Cannabis concentrates come in many forms with many different profiles, but the basics for turning raw plant material into an oil (or eventually into isolate, shatter, or wax) remain the same. Essentially, the goal is to strip the plant material of cannabinoids and terpenes while not drawing out chlorophyll and other undesirable plant compounds. Because the cannabinoids are removed from the plant and concentrated, very small volumes have the same effect as much larger volumes of the plant material.
Cannabinoids and terpenes are non-polar (a characteristic that makes them hydrophobic and “oily”), whereas many other plant molecules, like chlorophyll, are polar (which makes them hydrophilic and dissolvable in water). Figuring out how to extract the desirable compounds without pulling the undesirable ones is at the heart of the extraction debate. Processors should get clear on their extraction goals, as there is not a single method that works best. Each system has its strengths and weaknesses, and each lab should choose the system that integrates the best with their extraction goals, facility, expertise, and budget.
Hydrocarbon Extraction
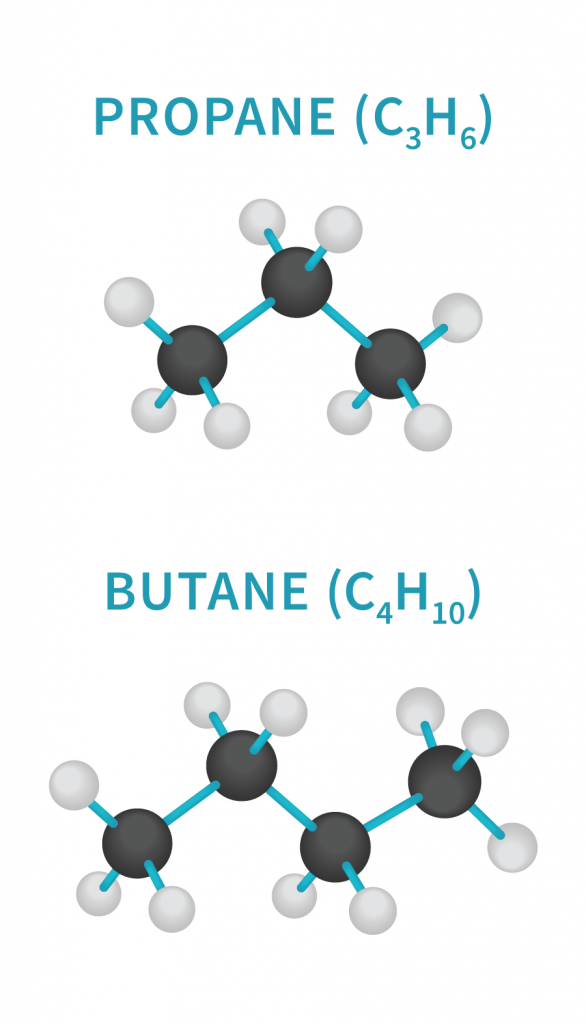
Hydrocarbons are molecules composed of only carbon atoms surrounded by hydrogen. They are defined by their number of carbons, ranging from methane (with only one carbon atom) on up. Butane, the most common hydrocarbon solvent, has four carbons, while another popular option, propane, has three. For extractions, these gases are pressurized and turned into liquids, which are then washed over the cannabis.
Hydrocarbons’ structure makes them non-polar, which allows them to extract other non-polar molecules (like cannabinoids and terpenes), while not attracting the plant’s polar parts (like the more aqueous alkaloids and chlorophyll). Butane and propane are easily evaporated from the solution, which is usually done in a vacuum oven. Hydrocarbon extraction should only be done by a reputable extractor who knows how to purge as much of the solvent as possible during this phase.
While a competent and experienced extractor and a state-of-the-art lab may bring residual butane levels down to less than 1%, trace amounts are always left. While these have been deemed generally safe, and some states have put levels on acceptable butane levels, the fact remains that the consumer is ingesting a toxic substance, even if only a small amount. Here at Boojum Group, we are acutely aware that as a CBD processor, we are providing products to some of the most vulnerable members of our community. People with compromised immune systems, chronic pain, cancer, and other ailments may need larger dosages than average, which means increased exposure to hydrocarbons if they are buying oil produced using this method.
Butane, when consumed regularly or in large amounts, has many side-effects, and is considered toxic to the central nervous system. With science still racing to catch up, and so much still unknown in the cannabis industry, adding a known neurotoxin to the mix is a risk that needs to be assessed by producers and manufacturers. If you are buying extract made with hydrocarbons, make sure to ask for lab results with residual solvent analysis and do your research to ensure that those levels pose as little risk as possible to your consumers or yourself.
Butane and propane are extremely flammable gasses, making hydrocarbon extraction more dangerous than other systems. New, closed-loop extractor systems keep these gasses in a protective environment (where they do not have a chance to mix with air, which makes them volatile) and where the butane can be condensed and recovered. Still, hydrocarbon extraction requires a C1D1 facility, and can be highly dangerous when conducted by inexperienced processors.
Despite the dangers of running hydrocarbon extractions and of consuming trace amounts of these gasses, these are popular due to a much lower cost of admission than other methods (about one fifth the up-front costs as CO2 extraction systems), and shorter extraction times. If you’re building out a hydrocarbon lab, be sure to factor in the additional costs of applicable fire and safety codes, and be diligent about meeting regulatory benchmarks and inspections, as well as ethical standards in dealing with your consumers.
CO2 Extraction
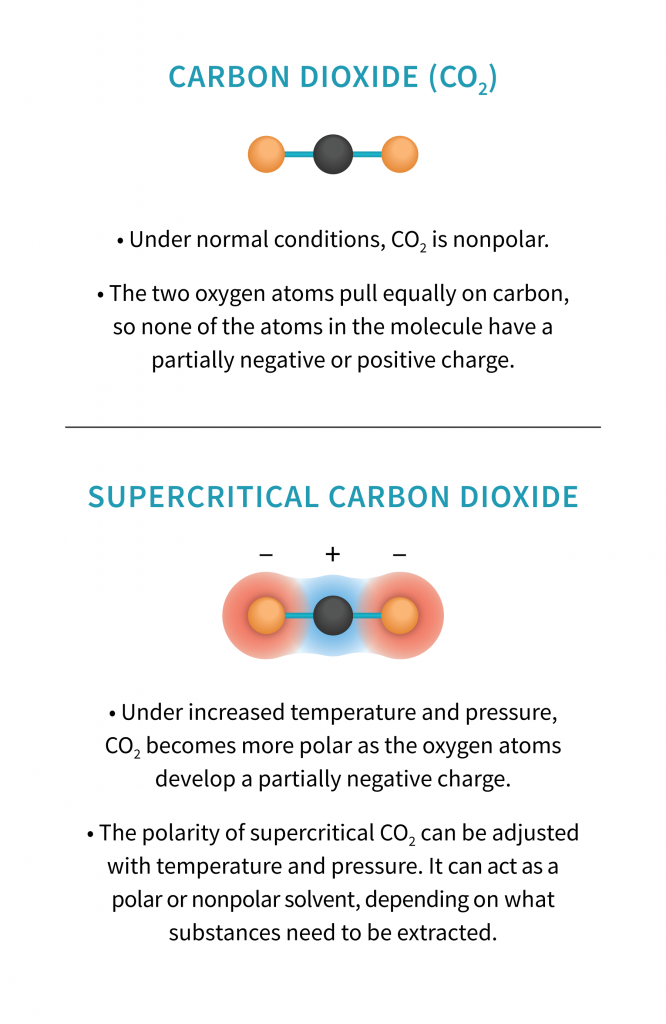
Whether or not you’re in the cannabis industry, you’re familiar with carbon dioxide, or CO2. We (along with all other aerobic organisms) breathe it out every time we exhale, drink it in carbonated beverages, and spray it out of fire extinguishers. You’ve probably also encountered it in its solid form, dry ice. You may not, however, recognize it in its supercritical phase, which it must be in for cannabis extraction. This phase, which can be reached using high temperatures and pressure, is somewhere between a gas and a clear liquid, and is important because it changes the polarity of the CO2. In other forms, CO2 is non-polar, but as you increase temperature and pressure, it becomes more polar, or hydrophilic.
CO2 solubility changes with polarity, so skilled extractors can adjust the pressure and temperature on advanced machines in order to pull out specific molecules with known polarities from the plant. This allows for fractioning—meaning that processors can target exact molecules to extract, like CBG or THCV. These changes can also affect the terpene concentrations pulled from the plant. For those looking for a very precise cannabinoid or profile in their oil, supercritical CO2 allows for more targeted extraction and a cleaner product-though it may come at the expense of the synergistic effects of full-spectrum oil.
Since using this process requires finely controlled pressure and temperature to hold CO2 in its supercritical stage, extraction machines are much more expensive than any other method, creating a high barrier of entry for many smaller companies or individuals. In addition to the cost of the extraction machines themselves, processors looking at this system should also take into account the high energy costs associated with it, as well as salary for skilled technicians.
These expensive machines, do, howerever, have an upside, in that their parameters tend to be more adjustable than other methods. Also, supercritical CO2 seems to kill any mold or bacteria in plant material, and using supercritical CO2 is a safe option, as it’s non-toxic and non-flammable.
Ethanol Extraction
Ethanol is the principle type of alcohol found in alcoholic drinks. Usually made from corn, it’s used as a cleaner, an antiseptic, a recreational drug, fuel, and of course, a solvent. Ethanol is composed of two different groups bound together. One group (the hydroxide, or OH group) is polar, and the other (the ethyl group, C2H5) is non-polar, making it effective at extracting both types of molecules.
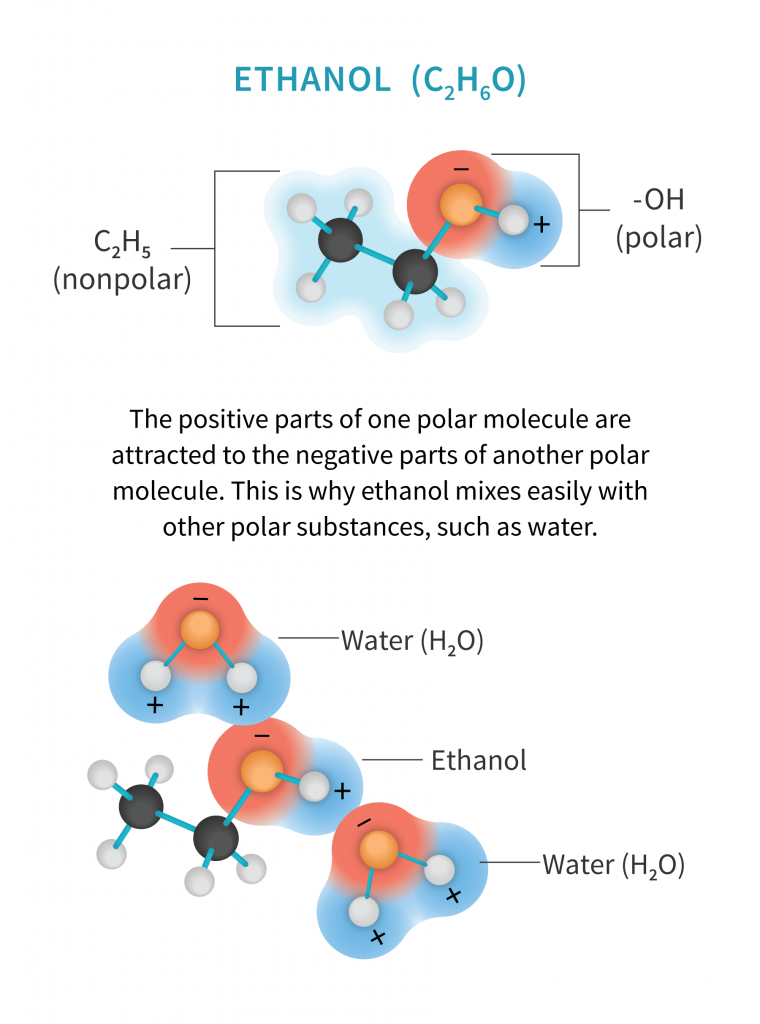
Ethanol’s strength is also its weakness, as consumers who are looking for an isolated product, or are pursuing a profile with solely cannabinoids and terpenes, might look elsewhere. However, as research progresses and the cannabis consumer becomes more informed, demand has grown for full-spectrum oil. Whole-plant benefits come from polar as well as non-polar molecules, so being able to extract both is helpful, even when it means extracting additional plant material that then needs to be removed.
This happens during the winterization process (which happens automatically when using cryogenic ethanol), when unwanted molecules freeze into solids, “precipitate” from the extract, and can be removed. Despite this removal process, ethanol extraction tends to leave a more full-bodied oil behind, with lower purity of individual cannabinoids, but a more natural profile of the whole plant.
Here at Boojum Group, we use 200-proof organic ethanol. We believe that full-spectrum oil is the most therapeutic product for our customers, and we love that ethanol is the best solvent to extract it. Since pure ethanol is safe for human consumption and non-toxic, we know that our product is both safe to work with in the lab, and safe for our customers to consume. Ethanol extraction uses less power than other methods, making it a win for the environment as well. Plus, we only use organically grown hemp, so making sure our solvent is safe and organic too is important to us.
Ethanol extraction is also unique because, unlike hydrocarbon and CO2 extraction equipment, ethanol does not require high pressure equipment and precautions. This means lower operating costs all around, and often less pushback from state and local regulators who are already familiar and comfortable with ethanol as a solvent – after all, humans have used it that way for millenia.

And the Winner is?
Well, we clearly have a favorite here at Boojum Group. We chose an ethanol extraction system based on the information above. We love working with organic material, and truly believe that it produces the best possible product for our consumers. The lower price point of ethanol extraction as compared to CO2, along with the higher throughput, were factors in our decision, along with the ability to draw a more robust, full-spectrum product from both polar and non-polar elements. While the process is more expensive than hydrocarbon extraction, we don’t believe that butane or propane has any place in a holistic and healthy product, and have chosen never to compromise the safety or trust of our consumers for profit.
That said, every lab is different, and in the end it all comes down to the goals and scope of the processor. There is no unanimous agreement on which extraction technique is the best, and it’s likely to stay that way. Each company will have different resources and budgets, unique production goals, and individual reasoning for using their extraction method of choice. When making your decision, it’s important to think about the type and quality of product you want to make and who you are making it for, the safety measures you can accommodate, the throughput you need, and the resources you have available to make it happen. Operated safely and correctly, hydrocarbon, supercritical CO2, and cryogenic ethanol extractions will all produce potent, high-quality concentrate.

Very good article. Well written and informative! Easy to read..
Thank you!
Someone in Ks hoping we get legal for recreational AND medicinal purposes nationwide!
Thanks for reading!
You must take part in a contest for top-of-the-line blogs on the web. I will suggest this site!
That would be amazing, thank you! 💚
Very well written and very informative. Thanks! I had no idea what toxins are in vapes processed with hydrocarbons. Definitely checking now before I buy!