For the first time in nearly a century, Utah patients have access to the potent, natural medicine of the entire cannabis plant.
The Utah Medical Cannabis Act allows patients to use THC along with CBD and all other cannabinoids to treat a list of qualifying conditions and provide relief from pain, nausea, inflammation, and a host of other symptoms. With close to 70% of the American population currently prescribed medication, cannabis offers alternatives and enhancements to the status quo.
While the addition of cannabis to a patient’s regimen has many benefits, it’s important to consider how cannabis will affect the body, specifically when it comes to how it interacts with other medications. If you are taking medication and would like to add cannabis to your regimen—whether in the form of CBD isolate, hemp extract, or medical marijuana—it’s a good idea to talk to your doctor about possible interactions. Products with CBD isolate and cannabis in the form of hemp extract contain less than 0.3% THC and can be purchased over the counter. But cannabis with more than 0.3% THC is considered medical cannabis in Utah and many other states, and requires a doctor’s prescription from a Qualified Medical Practitioner (QMP).
Because most studies on cannabinoids have been conducted using CBD or THC isolate, we don’t have a lot of information on drug interactions with whole-plant cannabis. If you read our article on the Entourage Effect, you know that cannabis contains hundreds of biomolecules (cannabinoids, terpenes, flavonoids, etc) that work in synergy with each other. Not only that, but cannabinoid and terpene ratios can differ greatly, depending on variety and cultivar.
Science has a long way to go before we have a more complete understanding of how cannabis interacts with various medications. Though with more and more research and resources directed towards minor cannabinoids and terpenes, our understanding of them and their interactions continues to grow and evolve. Both of the cannabinoid superstars, THC and CBD, have mountains of anecdotal evidence supporting their therapeutic qualities, to which scientific research and clinical studies are beginning to provide a framework of support.
How Safe is Cannabis?
Far more clinical and in vivo (which means inside a living organism) studies are needed to really understand how cannabis as a whole plant and also its isolated components, work in the body. But realistically, it will be a long time before clinical trials and research studies catch up to the anecdotal evidence on cannabis. While anecdotal evidence isn’t scientifically pertinent, there are tools we can use to examine the risk levels of consuming cannabis. The therapeutic index of cannabis gives us a good measurement to gauge the dangers of overdose. And as studies proliferate, researchers are gaining a better understanding of cannabis pharmacokinetics (how cannabinoids move through the body) and pharmacodynamics (how the body responds to cannabinoids).
Therapeutic Index: An Eye-Opening Metric
In medicine, drugs can be examined through a “therapeutic index”, or a ratio that compares the amount that would lead to overdose to the amount needed for therapeutic effect. This ratio gives a sense of how easily a drug can be dosed to be effective and avoid side effects. The higher the ratio, the easier it is to find that balance. THC’s therapeutic index has been estimated to be 40,000:1 (Barar, 2000). So in order to overdose, you’d have to consume at least forty thousand times a normal dose. For context, morphine’s therapeutic index is 70:1 (Williamson & Evans, 2000) and acetaminophen’s therapeutic index is ~3:1 (Bloom, 2017). Therapeutic index (TI) is an approximation of the safety margin between a therapeutic dose versus a lethal dose. It is only an indicative measure of the likelihood of a toxic dose, not a measure of absolute toxicity.

Diving Into Cannabis Pharmacology
Pharmacology—the study of the interactions between drugs and the body—is divided into two broad categories, pharmacokinetics and pharmacodynamics. Pharmacodynamics refers to the body’s biological response to a drug. Pharmacokinetics, meanwhile, looks at how drugs move through the body, and is concerned with their absorption, distribution, metabolism, and excretion. Dose size, concentration, timing, bioavailability, and intensity of effects all come into play (Tozer 2006). When we consume cannabis or other drugs, we can predict how it will react and interact with other substances based on how the body processes it.
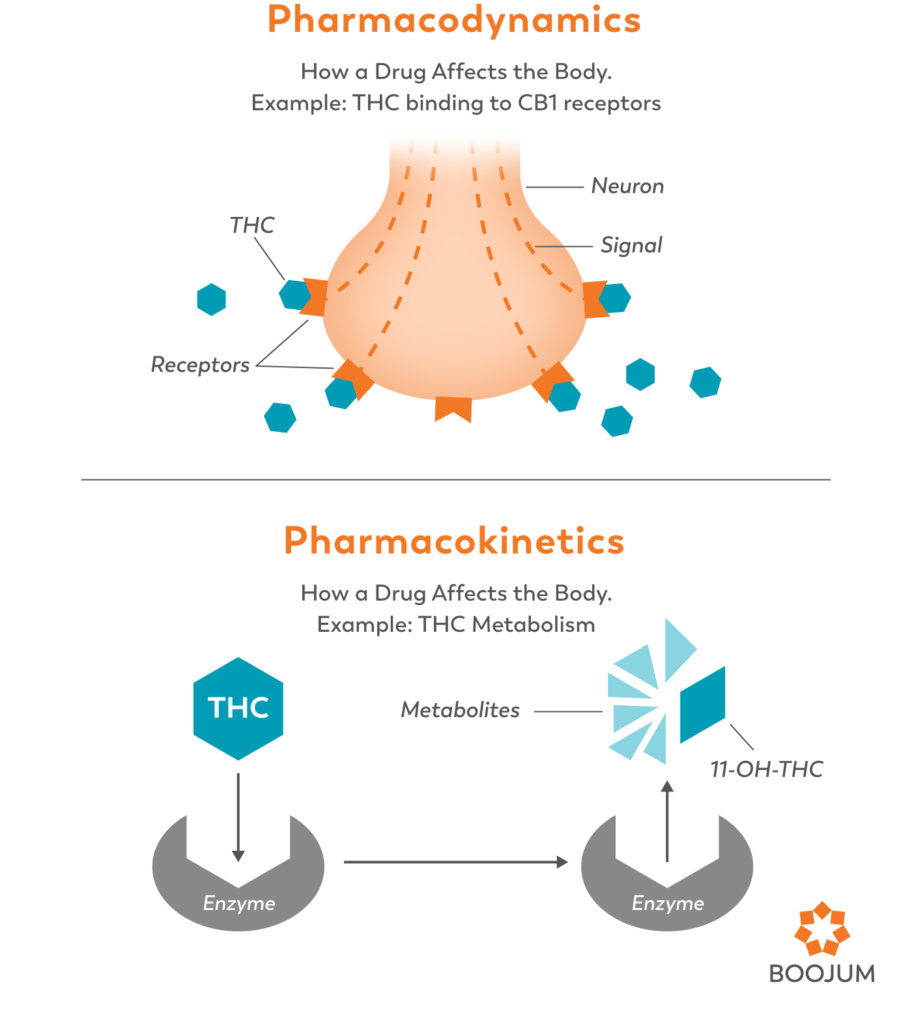
Pharmacodynamics of Cannabis
Simply put, pharmacodynamics refer to how cannabinoids act on the body. The entire endocannabinoid system was discovered through the study of pharmacodynamics. In an attempt to learn how THC was exerting its psychoactive effects, Raphael Mechoulam researched and found the receptors that it activated (CB1). He was soon able to show that these receptors were part of an extensive system of receptors and the molecules that engage them (Mechoulam 1998). These molecules are now well known as cannabinoids (both the endocannabinoids that our bodies produce, and the phytocannabinoids from cannabis) and the receptors they activate are known as cannabinoid receptors.
Pharmacokinetics of Cannabis
Pharmacokinetics refers to how the body acts on a drug and moves it through the body. While pharmacokinetics is responsible for many factors of how drugs are broken down, absorbed, and eliminated, the part of the process that is of particular interest when it comes to drug interactions is metabolism. Enzymes in the liver are responsible for metabolizing about 90% of the drugs on the market today, including cannabis. Enzymes bind to a substrate (in this case, the target drug) and break it down into metabolites. The more efficient the enzymes are, the milder the effects of a given drug are, and the quicker it will leave your system. Other enzymes act more slowly in breaking down the substrate, allowing it to circulate and affect the body for longer periods of time.
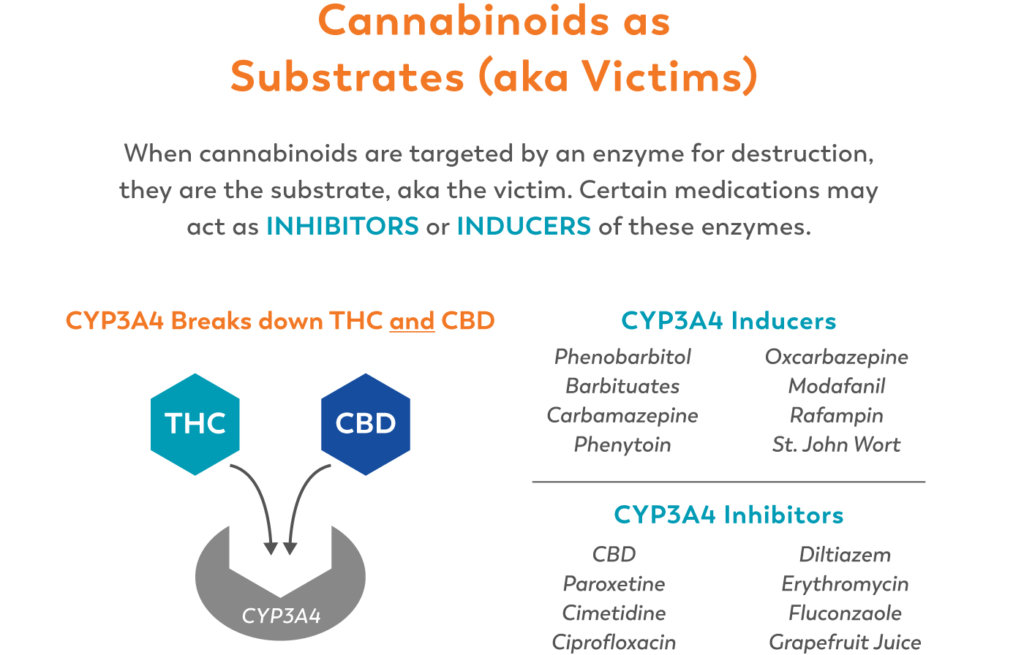
Cannabinoids as Victims
Drugs of all kinds compete at these enzymes, and are always either the “victim” or the “perpetrator” in drug-drug interactions. When drugs are the “victim”, they are the substrate, or the target that the enzyme will break down. When they are the “perpetrator”, they are either inducing or inhibiting the enzymes. When an enzyme is inhibited, it metabolizes much slower, leading to higher levels of the “victim” drug circulating in the body, and thus longer and more intense effects. When an enzyme is induced, it works more efficiently, clearing away the “victim” drug more quickly and minimizing effects.
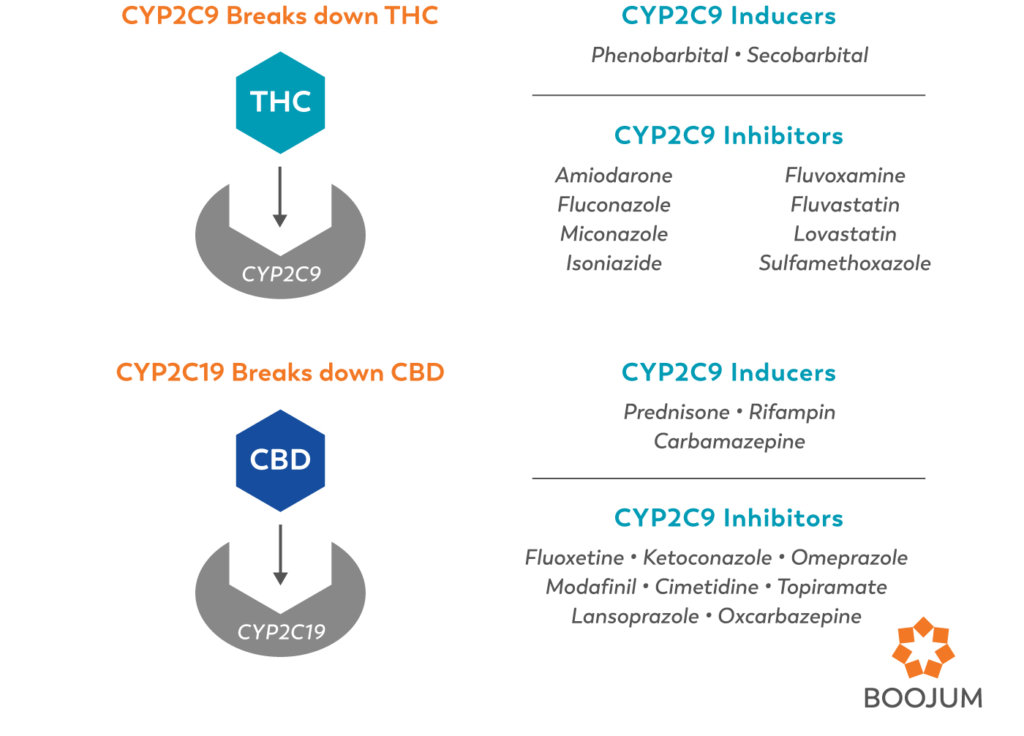
So, for example, ∆9-THC enters the liver, where it (serving as the substrate) is grabbed by the (CYP)2C9 enzyme and metabolized into 11-OH-THC. Many drugs (including most NSAIDS, like ibuprofen) are (CYP)2C9 inhibitors. In this role, the inhibitors make the enzymes less efficient, so they take longer to metabolize the THC, which would theoretically mean a longer-lasting and more psychoactive high.
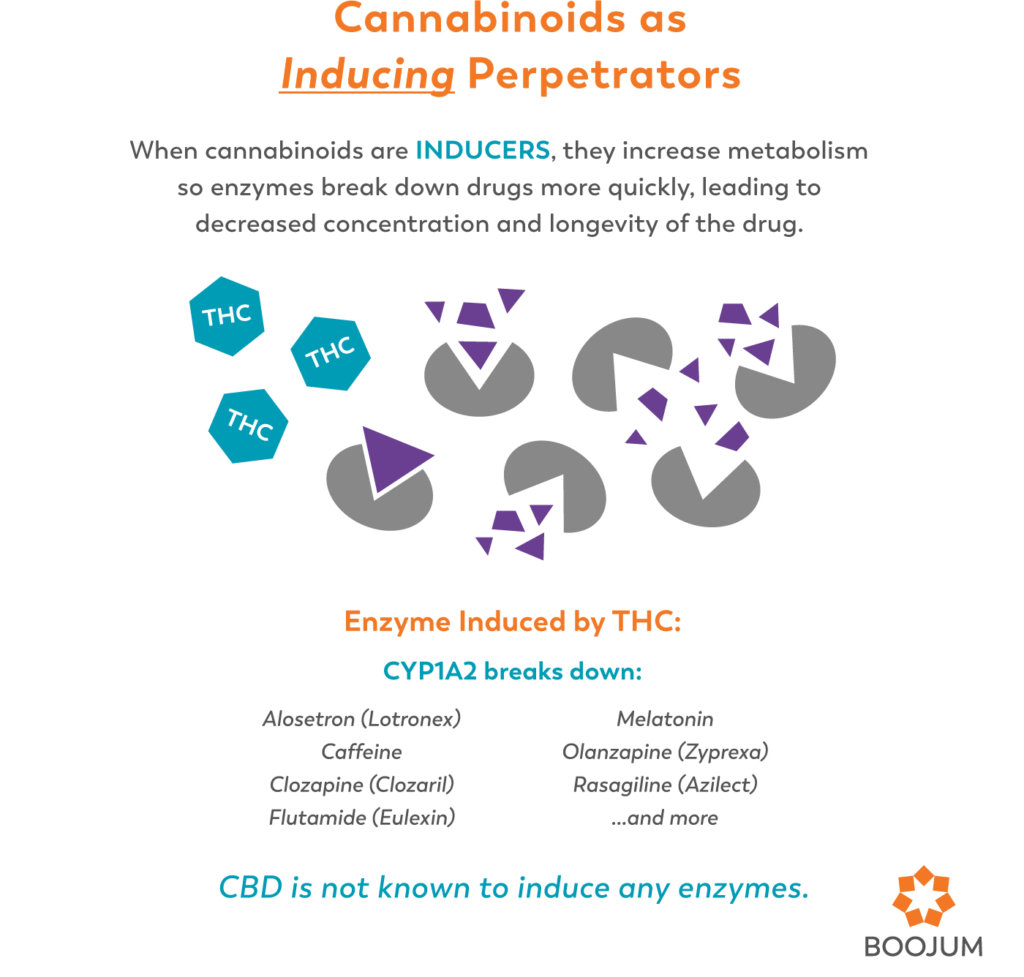
Cannabinoids as Perpetrators
When cannabinoids aren’t acting as the substrate for enzymes, they can work as inhibitors themselves. CBD is considered a potent inhibitor of (CYP)3A4 and (CYP)2D6 enzymes, and THC is an inducer of CYP1A2 (Ingelman-Sundberg, 2007). So when taking cannabis along with prescription drugs, take into account that any drugs metabolized by (CYP)3A4 or (CYP)2D6 will be more potent (and have potentially greater side effects and toxicity), while drugs metabolized by CYP1A2 may be less effective (table 2).

Always Ask your Doctor!
While science has given us some vital clues as to how cannabis may interact with certain drugs, there isn’t much hard data to go on. So while cannabinoids are considered to be generally well tolerated, with little adverse reaction, it is always advisable to speak to your doctor about changes or additions to your medication.
Resources
Alsherbiny, M. A., & Li, C. G. (2018). Medicinal Cannabis-Potential Drug Interactions. Medicines (Basel, Switzerland), 6(1), 3. https://doi.org/10.3390/medicines6010003
Barar, F. S. K. (2000). Essentials of pharmacotherapeutics. S. Chand Publishing.
Bland, T.M., Haining, R. L., Tracy, T. S., & Callery, P. S. (2005) CYP2C-catalyzed delta9-tetrahydrocannabinol metabolism: kinetics, pharmacogenetics and interaction with phenytoin. Biochemical Pharmacology, 70(7), 1096-1103
Bloom, J. 2017, September 11. “Is Tylenol ‘By Far the Most Dangerous Drug Ever Made?’”. https://www.acsh.org/news/2017/09/11/tylenol-far-most-dangerous-drug-ever-made-11711
Cox, Emily J, Maharao, Neha, Patilea-Vrana, Gabriela, Unadkat, Jashvant D, Rettie, Allan E, McCune, Jeannine S, & Paine, Mary F. (2019). A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics. Pharmacology & Therapeutics., 201, 25-38.
D’Souza, D., Cortes-Briones, J., Ranganathan, M., Thurnauer, H., Creatura, G., Surti, T., & Carson, R. (2015, December). Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Neuropsychopharmacology (Vol. 40, pp. S589-S590).
Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007 Dec;116(3):496-526. Epub 2007 Oct 9. Review. PubMed: 18001838.
Lucas, C. J., Galettis, P., & Schneider, J. (2018). The pharmacokinetics and the pharmacodynamics of cannabinoids. British journal of clinical pharmacology, 84(11), 2477-2482.
Mechoulam, R., Fride, E., & Di Marzo, V. (1998). Endocannabinoids. European journal of pharmacology, 359(1), 1-18.
Nahtigal, I.G., Blake, A., Hand, A., Florentinus-Mefailoski, A., Hashemi, H., & Friedberg, J. (2017). The pharmacological properties of cannabis.
Sim-Selley, L. J., Schechter, N. S., Rorrer, W. K., Dalton, G. D., Hernandez, J., Martin, B. R., & Selley, D. E. (2006). Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Molecular pharmacology, 70(3), 986-996.
Stott, C., White, L., Wright, S., Wilbraham, D., & Guy, G. (2013). A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus, 2(1), 236.
Tozer, T. N., & Rowland, M. (2006). Introduction to pharmacokinetics and pharmacodynamics: the quantitative basis of drug therapy. Lippincott Williams & Wilkins.
Williamson, E. M., & Evans, F. J. (2000). Cannabinoids in clinical practice. Drugs, 60(6),
1303–1314. https://doi.org/10.2165/00003495-200060060-00005
Zendulka, O., Dovrtělová, G., Nosková, K., Turjap, M., Šulcová, A., Hanuš, L., & Juřica, J. (2016). Cannabinoids and Cytochrome P450 Interactions. Current drug metabolism, 17(3), 206–226. https://doi.org/10.2174/1389200217666151210142051

I think my biggest question is ..
Does this mean CBD’s inhibition with enzyme CYP2D6 breaks down codeine / hydrocodone slower? … thus rendering a prolonged but less intensified effect of the opiate?
And does this mean that THC reduces the prevalence of testosterone?
Thnx
Thanks for reading!
CBD inhibits CYP2D6, meaning that its presence is likely to slow the metabolism of codein. While this increases codein’s presence (both in terms of the strength and longevity of those molecules, as it isn’t being quickly metabolized), it decreases its metabolites. Since the analgesic benefits of codein come from the active metabolites, which there are subsequently fewer of, we’d expect a lessened effect. As far as testosterone, rats treated with CBD showed inhibition of testosterone oxidation in the liver (meaning more testosterone and less metabolites). Research is needed (and in some cases happening!) on whether that translates to humans and to what extent these inhibitions and inductions translate to real world benefit or conflicts.